cGMP SUBSTRATES
Experiment confident clinical trials with cGMP grade substrates !
For more than 30 years Euriso-top has been manufacturing non-radioactive stable isotope labeled compounds (carbon-13, deuterium, nitrogen-15, oxygen-18).
In 2020, the ANSM (French national agency for the safety of medicinal and health products), the competent authority in France, renewed our certificate of Good Manufacturing Practice (EU GMP) compliance as a manufacturer for a part of our catalog.
Euriso-Top has manufactured bulk active pharmaceutical ingredients (APIs) since 1994. It recently added a new facility complex, right in the middle of the CEA Saclay, to complement its existing cGMP facilities.
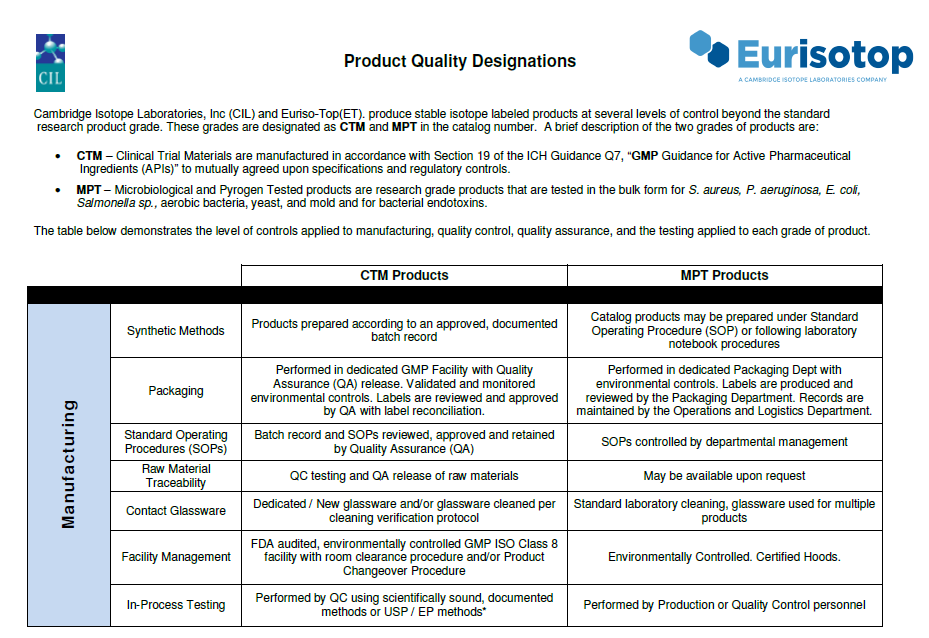
Quality Control
All our active substances are analyzed according to internal monographs and European Pharmacopoeia methods. Chemical and biological analyses are performed at different stages of the manufacturing process:
- Chemical purity ( ⩾ 98% and/or 99%) : HPLC, 13C-NMR, 1 H-NMR, assay of European Pharmacopoeia (Eur. Ph.)
- Isotopic enrichment ( ⩾ 98% and/or 99%) : GC/MS, LC/MS, 1 H-NMR
- Residual solvents : GC (Head space), Eur. Ph. (2.2.28)
- Endotoxins : LAL test Eur. Ph. (2.6.14)
- Bioburden : Eur. Ph (2.6.12)
- Heavy Metals : Eur. Ph. (2.4.8)
- Water content : Karl Fisher, Eur. Ph. (2.512)
- Other assays of the Eur. Ph.
Related products
Technical Products Information